Application research of 4-Methylpyridin-2-amine
Introduction
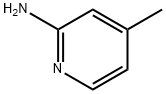
4-Methylpyridin-2-amine (Figure 1) is a pyridine organic compound containing amino and methyl substituents. It is an important chemical raw material and is widely used in medicine, pesticide and other fields.

Application Research example
A potent inhibitor of inducible NO synthase activity in vitro and in vivo
The ability of 4-methylpyridin-2-amine to inhibit the catalytic activity of the inducible NO synthase (NOS II) enzyme was characterized in vitro and in vivo. In vitro, 4-methylpyridin-2-amine inhibited NOS II activity derived from mouse RAW 264.7 cells with an IC50 of 6 nM. Enzyme kinetic studies indicated that inhibition is competitive with respect to arginine. 4-Methylpyridin-2-amine was less potent on human recombinant NOS II (IC50 = 40 nM) and was still less potent on human recombinant NOS I and NOS III (IC50 = 100 nM). NG-monomethyl-L-arginine (L-NMMA), N6-iminoethyl-L-lysine (L-NIL) and aminoguanidine were much weaker inhibitors of murine NOS II than 4-methylpyridin-2-amine but, unlike 4-methylpyridin-2-amine, retained similar activity on human recombinant NOS II. L-NMMA inhibited all three NOS isoforms with similar potency (IC50S 3-7 microM). In contrast, compared to activity on human recombinant NOS III, L-NIL displayed 10 x selectivity for murine NOS II and 11 x selectivity for human recombinant NOS II while aminoguanidine displayed 7.3 x selectivity for murine NOS II and 3.7 x selectivity for human recombinant NOS II. Mouse RAW 264.7 macrophages produced high levels of nitrite when cultured overnight in the presence of lipopolysaccharide (LPS) and interferon-gamma. Addition of 4-methylpyridin-2-amine at the same time as the LPS and IFN-gamma, dose-dependently reduced the levels of nitrite (IC50 = 1.5 microM) without affecting the induction of NOS II protein. Increasing the extracellular concentration of arginine decreased the potency of 4-methylpyridin-2-amine but at concentrations up to 10 microM, 4-methylpyridin-2-amine did not inhibit the uptake of [3H]-arginine into the cell. Addition of 4-methylpyridin-2-amine after the enzyme was induced also dose-dependently inhibited nitrite production. Together, these data suggest that 4-methylpyridin-2-amine reduces cellular production of NO by competitive inhibition of the catalytic activity of NOS II, in agreement with results obtained from in vitro enzyme kinetic studies. When infused i.v. in conscious unrestrained rats, 4-methylpyridin-2-amine inhibited the rise in plasma nitrate produced in response to intraperitoneal injection of LPS (ID50 = 0.009 mg kg-1min-1). Larger doses of 4-methylpyridin-2-amine were required to raise mean arterial pressure in untreated conscious rats (ED50 = 0.060 mg kg-1 min-1) indicating 6.9 x selectivity for NOS II over NOS III in vivo. Under the same conditions, L-NMMA was nonselective while L-NIL and aminoguanidine displayed 5.2 x and 8.6 x selectivity respectively. All of these compounds caused significant increases in mean arterial pressure at doses above the ID50 for inhibition of NOS II activity in vivo. 4-Methylpyridin-2-amine also inhibited LPS-induced elevation in plasma nitrate after either subcutaneous (ID50 = 0.3 mg/kg) or oral (ID50 = 20.8 mg/kg) administration. These data indicate that 4-methylpyridin-2-amine is a potent inhibitor of NOS II activity in vitro and in vivo with a similar degree of isozyme selectivity to that of L-NIL and aminoguanidine in rodents.[1]
2. [18F]6-(2-Fluoropropyl)-4-methyl-pyridin-2-amine.
In the present study, 4-methylpyridin-2-amine was reacted with 3-bromothiophene-2-carbaldehyde and the Schiff base (E)-1-(3-bromothiophen-2-yl)-N-(4-methylpyridin-2-yl)methanimine was obtained in a 79% yield. Coupling of the Schiff base with aryl/het-aryl boronic acids under Suzuki coupling reaction conditions, using Pd(PPh3) 4 as catalyst, yielded products with the hydrolysis of the imine linkages ( 5a- 5k, 6a- 6h) in good to moderate yields. To gain mechanistic insight into the transition metal-catalyzed hydrolysis of the compounds, density functional theory (DFT) calculations were performed. The theoretical calculations strongly supported the experiment and provided an insight into the transition metal-catalyzed hydrolysis of imines.[2]
3.Influence of 4-methylpyridin-2-amine and 2-Aminopyrimidine Ligands on the Malonic Acid-Cu(II) System
Two Cu(II)-malonate complexes with 4-methylpyridin-2-amine (complex 1) and 2-aminopyrimidine (complex 2) auxiliary ligands were synthesized, and their single-crystal X-ray diffraction structures were established. Change in the auxiliary ligand exhibits substantial structural variation in the present complexes. Complex 1 shows a one-dimensional anionic copper-malonate moiety connected by the malonate bridge, whereas complex 2 is a mononuclear one. For both the complexes, auxiliary ligands are attached with the Cu-malonate moiety through various noncovalent interactions. Optical band gap, electrical conductivity, and photosensitivity of complexes 1 and 2 were measured, but the values of electrical parameters of the complexes significantly differ from each other. However, the magnitudes of electrical parameters increase several times for both the complexes when they are exposed under visible light, though the values of light sensing parameters of complex 1 were found to be higher than those of complex 2. Density functional theory calculations for complex 1 were carried out to support the experimental result.[3]
4.On the mechanism of action of 4-methylpyridin-2-amine
2-Amino-4-methylpyridine (2-AMP), when implanted into the ventral lateral thalamus of rats, does not cause stereotyped behavior. On the other hand, when compulsive biting and gnawing was evoked by thalamic implants of morphine (via stimulation of type 1 opiate receptors, which activate a dopaminergic mechanism) or of apomorphine, systemic application of 2-AMP suppressed this response. The anti-gnawing effect of 2-AMP was abolished by naloxone, indicating that the inhibitory action of 2-AMP involves activation of another, viz. type 2 receptor. The antagonistic influence of 2-AMP could also be suppressed by pretreatment of the rats with p-chlorophenylalanine. It is concluded that 2-AMP has only a weak effect on type 1 opiate receptors, linked to a dopaminergic mechanism, but it possesses high affinity to type 2 opiate receptors, which are connected with serotonergic pathways.[4]
5.Inhibition of nitric oxide synthase aggravates cisplatin-induced nephrotoxicity
Nitric oxide (NO) has been shown to play a role in maintaining normal renal function. However, the role of NO in cisplatin (CDDP)-induced nephrotoxicity is still unclear. The aim of the present work was to examine the effect of the NO synthase (NOS) inhibitor, 4-methylpyridin-2-amine, on the severity of CDDP-induced nephrotoxicity. Methods: Male Wistar rats were divided into six groups. Three control groups received plain drinking water or water containing 1.5% L-arginine. One of the two groups receiving plain water was treated with an intraperitoneal injection of 4-methylpyridin-2-amine (1 mg/kg in normal saline), and the other two control groups were injected intraperitoneally with normal saline. Another three groups were treated in the same manner and injected with CDDP (6 mg/kg, i.p.). CDDP was injected 1 h after 4-methylpyridin-2-amine treatment. Rats were sacrificed 7 days after CDDP treatment, and serum as well as kidneys were isolated and analysed. Results: CDDP-treated rats showed increases in the kidney weight as a percentage of the total body weight and serum creatinine and urea levels and decreases in serum albumin and calcium levels. Also, CDDP treatment induced reductions in the kidney total nitrate/nitrite (NO(x)), reduced glutathione (GSH) and glutathione peroxidase activity (GSH-Px) levels and an increase in the kidney malondialdehyde (MDA) production level. In contrast, 4-methylpyridin-2-amine treatment 1 h prior to CDDP injection induced marked exacerbation of CDDP-induced nephrotoxicity, as manifested by severe aggravation of the indices of nephrotoxicity. Also, 4-methylpyridin-2-amine plus CDDP-treated rats showed exaggeration of the reduction in the kidney total NO(x) content and GSH-Px activity and elevation of the kidney platinum accumulation level with normalization of the kidney MDA production level and rebound in the kidney GSH content. Histopathologically, CDDP-treated rats showed marked interstitial nephritis, tubular atrophy and tubular necrosis. However, treatment with 4-methylpyridin-2-amine 1 h prior to CDDP injection revealed marked exacerbation of CDDP-induced histopathological changes. Conclusions: The present findings suggest that NO plays a role in CDDP-induced nephrotoxicity. Administration of 4-methylpyridin-2-amine, an NOS inhibitor, exacerbates CDDP-induced nephrotoxicity.[5]
References
1. Faraci WS, Nagel AA, Verdries KA, et al. 2-Amino-4-methylpyridine as a potent inhibitor of inducible NO synthase activity in vitro and in vivo. Br J Pharmacol. 1996;119(6):1101-1108. doi:10.1111/j.1476-5381.1996.tb16010.x
2. Ahmad G, Rasool N, Rizwan K, et al. Role of Pyridine Nitrogen in Palladium-Catalyzed Imine Hydrolysis: A Case Study of (E)-1-(3-bromothiophen-2-yl)-N-(4-methylpyridin-2-yl)methanimine. Molecules. 2019;24(14):2609. Published 2019 Jul 17. doi:10.3390/molecules24142609
3. Mandal T, Dey A, Seth SK, et al. Influence of 2-Amino-4-methylpyridine and 2-Aminopyrimidine Ligands on the Malonic Acid-Cu(II) System: Insights through Supramolecular Interactions and Photoresponse Properties. ACS Omega. 2019;5(1):460-470. Published 2019 Dec 31. doi:10.1021/acsomega.9b02971
4.Bergmann F, Elam R. On the mechanism of action of 2-amino-4-methylpyridine, a morphine-like analgesic. Arch Int Pharmacodyn Ther. 1980;247(2):275-282.
5.Saad SY, Najjar TA, Daba MH, Al-Rikabi AC. Inhibition of nitric oxide synthase aggravates cisplatin-induced nephrotoxicity: effect of 2-amino-4-methylpyridine. Chemotherapy. 2002;48(6):309-315. doi:10.1159/000069714
You may like
See also
Lastest Price from 4-Methylpyridin-2-amine manufacturers

US $0.00/KG2025-04-21
- CAS:
- 695-34-1
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month
US $1.00/KG2025-04-21
- CAS:
- 695-34-1
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt